Organic Compound That Would Not Absorb Uv Vis Radiation
It is inexpensive and emails continuous radiation in. Organic compounds can also be classified or subdivided by the presence of heteroatoms eg organometallic compounds which feature bonds between carbon and a metal and organophosphorus compounds which feature bonds between carbon and a phosphorus.

Interpreting Uv Spectra Mcc Organic Chemistry
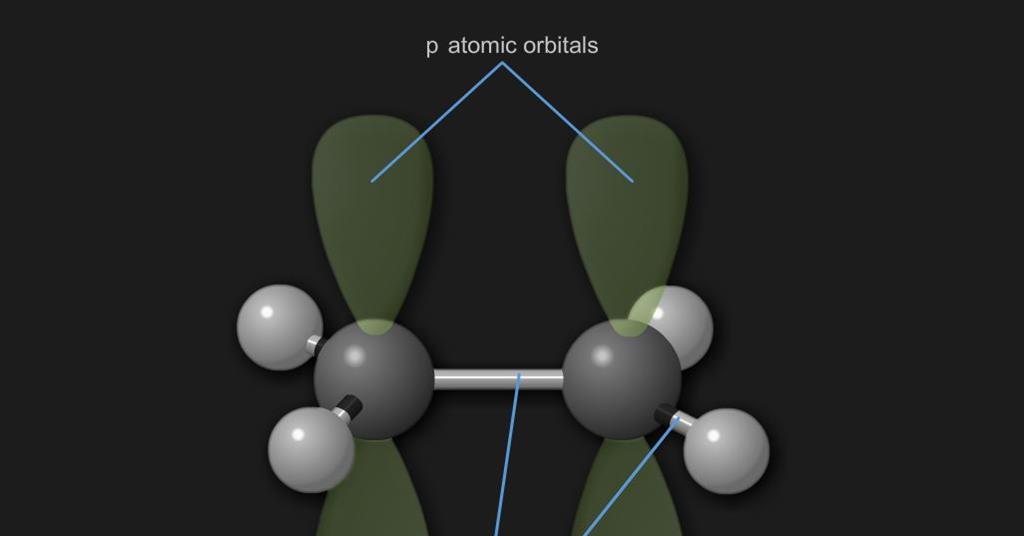
Ultraviolet Visible Spectroscopy Uv Vis The Origin Of Colour In Organic Compounds Resource Rsc Education
Uv Visible Spectroscopy
The diagram below shows a beam of monochromatic radiation of radiant power P 0 directed at a sample solutionAbsorption takes place and the beam of radiation leaving the sample has radiant power P.

Organic compound that would not absorb uv vis radiation. Exhibit regular shift towards longer wavelength Red shift Azo Compounds with the linkage NN- show low intensity bands in the near Uv and Vis due to n to transitions Azobenzenes absorb at about 445 nm the. Fortunately the higher energetic part of UV-B. 280 295 nm is filtered by the stratosphere and does not reach the earths surface UV-A 315 400 nm has energy between 389 and 300 KJ mol-1 and is less harmful for organic materials than UV-B.
It derives from a benzoic acidIt is a conjugate acid of a 4-aminobenzoate. One major distinction is between natural and synthetic compounds. Some organic molecules can absorb electromagnetic radiation in the form of photons of UV and Visible light.
Thiophene is a monocyclic heteroarene that is furan in which the oxygen atom is replaced by a sulfurIt has a role as a non-polar solvent. The solvents for these determinations are often water for water soluble compounds or ethanol for organic soluble compounds. As a function of wavelength UV-vis spectrophotometers measure the absorption or.
Tungsten filament lamp is the most commonly used source for visible radiation. The efficiency of direct UV irradiation or photolysis depends on the reactivity of the compound source of UV light water pH and temperature to remove MPs ranging from 0 to 100. Radiation with a range of wavelengths is directed through a sample of interest and a detector records which wavelengths were absorbed and to what extent the.
Xenon lamp may also be used for UV radiation but the radiation produced is not as stable as the hydrogen lamp. Whenever a coating extrudate or film is used it should not be UV transparent but should rather be pigmented to absorb in the 300-450 nm. UV-resistant additives can also be incorporated into the fibers to increase the fibers resistance to UV radiation.
PDF Organic Chemistry by David Klein pdf download 3rd. UV-VIS Detectors have mostly used detectors due to its specific response to the class of compounds or particular compounds depending on the functional groups of eluting molecules that absorb light although some compounds with no light absorbing groups give suitable response after post-column derivatization to introduce light absorbing entities. For example if you suspect an individual is hiding heroin in sugar you can use UVVIS spectroscopy to not only identify the heroin but also quantify it since the sugar does not.
Many compounds absorb ultraviolet UV or visible Vis light. Ultraviolet UV is a form of electromagnetic radiation with wavelength from 10 nm with a corresponding frequency around 30 PHz to 400 nm 750 THz shorter than that of visible light but longer than X-raysUV radiation is present in sunlight and constitutes about 10 of the total electromagnetic radiation output from the SunIt is also produced by electric arcs and specialized lights. Location of Components The spots produced by paper or thin-layer chromatography may be located by.
They absorb at three bands. The photocurrent produced by Sb 2 S 3 doped TiO 2 NTs is always higher than the photocurrent produced by the bare TiO 2 NTs if both are utilizing the same kind of excitation light UV or UVvis. Organic compounds may be classified in a variety of ways.
Typically the wavelength range used in UV detection for HPLC is in the range 200 400nm which covers both UV and the lower part of the visible spectrum. OR The functional group with non-bonding electrons that does not absorb radiation in near UV region but when attached to a chromophore alters the wavelength intensity of absorption. As mentioned on one Reagent Friday back in the day ozone does more than absorb UV radiation in the upper atmosphere and cause breathing problems in traffic-clogged cities.
As per the Beer-Lambert law the greater the number of absorbing molecules that have the ability to absorb light of a specific wavelength the greater the extent of absorption of the radiation. Ozone O 3 Is A Powerful Oxidant For Cleaving Alkenes To Carbonyl Compounds. Solutions of transition metal ions can be coloured ie absorb visible light because electrons within the metal atoms can be.
Organic compounds especially those with a high degree of conjugation also absorb light in the UV or visible regions of the electromagnetic spectrum. 1 direct inspection if the compounds are visible under white or either short-wavelength 254 nm or long-wavelength 360 nm UV light 2 inspection in white or UV light after treatment with reagents that will make the spots visible reagents are most conveniently applied with an atomizer. To learn more about the principle of UV-Visible spectroscopy and other related concepts such as infrared spectroscopy register with BYJUS and download the mobile application on your smartphone.
OR The functional group with non-bonding electrons that does not absorb radiation in near UV region but when attached to a chromophore alters the wavelength intensity of absorption. Similarly as in the case of B doped TiO 2 NTs the importance of the annealing heat treatment cannot be neglected to make effective the photoelectrocatalytic properties 3. We have been talking in general terms about how molecules absorb UV and visible light now lets look at some actual examples of data from a UV-vis absorbance spectrophotometer.
From the location of peaks and combination of peaks it can be concluded that whether the compound is saturated or unsaturated hetero atoms are present or not etc. UVVIS spectroscopy is also used for determining the concentration of illegal substances in mixtures. Sources of Visible radiation.
It is a mancude organic heteromonocyclic parent a member of thiophenes a monocyclic heteroarene and a volatile organic compound. UVVis spectroscopy is routinely used in the quantitative determination of solutions of transition metal ions and highly conjugated organic compounds. 4-aminobenzoic acid is an aminobenzoic acid in which the amino group is para to the carboxy group.
Benzene λmax255 nm Phenol λmax270 nm Aniline λmax280 nm 10. It has a role as an Escherichia coli metabolite a plant metabolite and an allergen. Ozone O 3 is the strongest oxidizing agent for the degradation of MPs because ozone reacts very quickly with MPs that have accessible amino groups double bonds or aromatic groups.
Its a powerful oxidant and since its discovery in the mid 1800s by Schönbein has found use in the cleavage of carbon-carbon. UV-vis spectroscopy is a cost-effective simple versatile non-destructive analytical technique suitable for a large spectrum of organic compounds and some inorganic species. The basic setup is the same as for IR spectroscopy.
Quantitative analysis UV absorption spectroscopy can be used for the quantitative determination of. Benzene λmax 255 nm OH Phenol λmax 270 nm NH2 Aniline λmax 280 nm. 260 200 and 180 nm Policyclic aromatic Naphthalene.
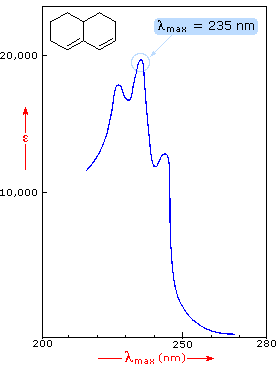
Uv Visible Spectroscopy

Solved 1 4 Points For What Does The Acronym Lumo Stand Chegg Com
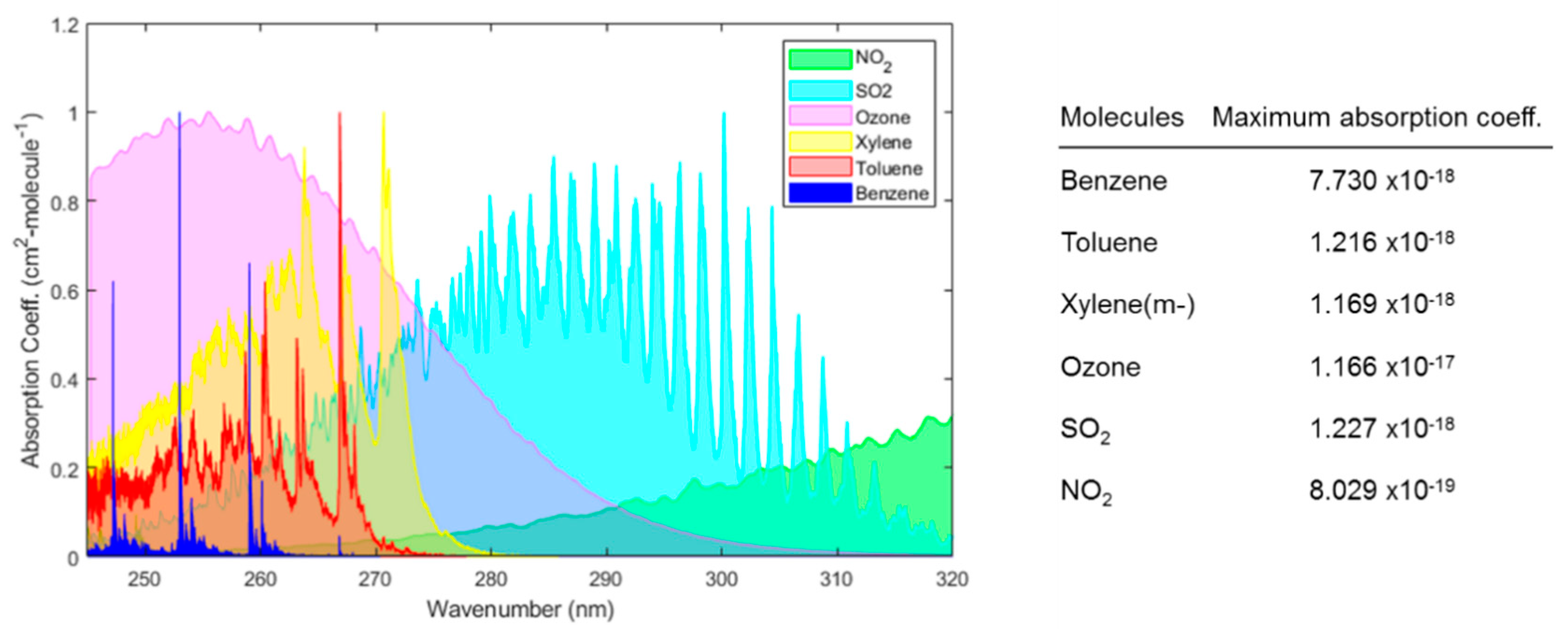
Sensors Free Full Text Gas Detection Using Portable Deep Uv Absorption Spectrophotometry A Review
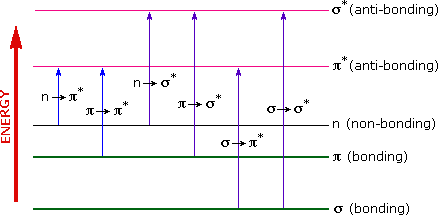
Uv Visible Spectroscopy
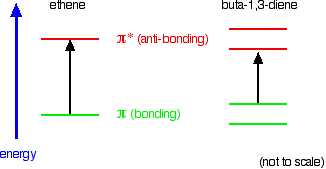
What Causes Molecules To Absorb Uv And Visible Light Chemistry Libretexts

Explain Origin Of Uv Visible Absorption Spectrum Through It What Information May Be Sought Regarding Structure Of Organic Compounds Pharmatutor
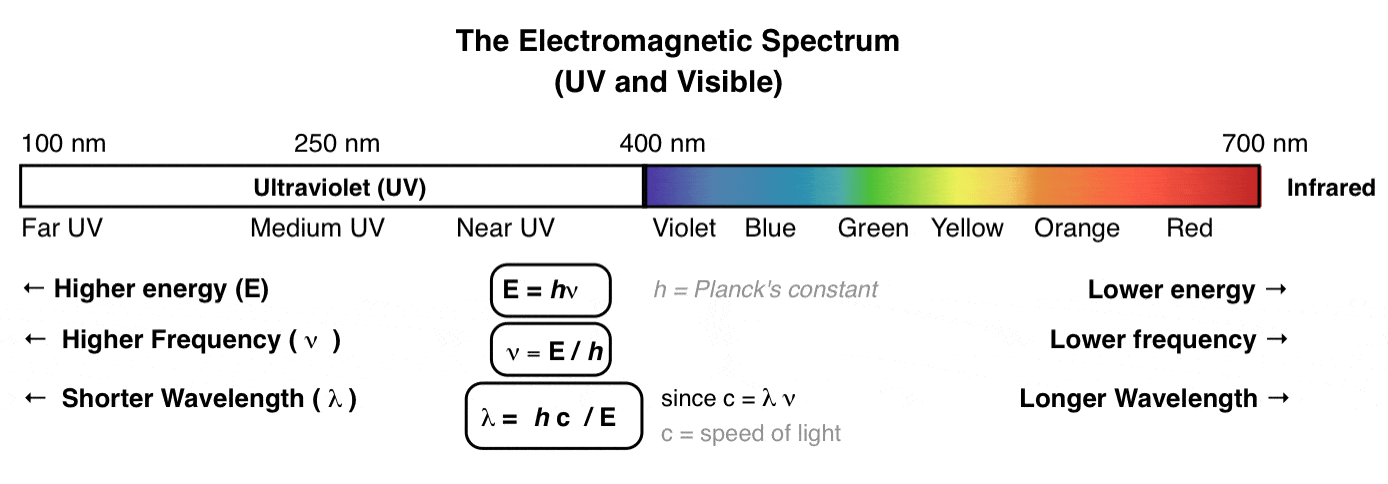
What Is Uv Vis Spectroscopy And How Does It Apply To Conjugation
1